Session Coordinator Course
/in /by Alex Ashton
Welcome to your Session Coordinator Training Course. Thank you for supporting us. This course has been put together to provide the theory training to augment your practical on-site training. It is vitally important that you work to our Standard Operating Procedures and to the principles of Good Manufacturing Practice (GMP) whilst working in your role.

If you are unsure of any of the training please do not worry and ask one of the team for help.
Work your way through the lessons as instructed by the Donor Admin Manager. To read a document click on the link which will take you to our secure document site.
Once you have read the document click on the back arrow in the top left-hand corner and this will bring you back to the Learndash lesson.
Once you have read the documents and completed any knowledge check exercises you can then move onto the next lesson. The training ends with a quiz which requires 100% pass mark. You have 2 attempts at the quiz but if you don’t pass please contact cpd@petbloodbankuk.org for assistance.
Please remember that this is an “open book” quiz so you can bookmark the Standard Operating Procedure documents as you work through the lessons and refer back to them, and the linked documents, as you work through the quiz.

Collection Kit Assistant
/in /by Alex Ashton
Collection Kit Assistant
Welcome to your new position within Pet Blood Bank, it is great you are working with us in our life-saving work.
As a Blood Bank we are governed by a branch of the government called the Veterinary Medicines Directorate or VMD. We have a long series of regulations we must adhere to in order to maintain our blood banking license and continue to save lives. Training is a large part of the VMD governance around which there are lots of rules.
This training course contains multiple lessons, some of which have been further subdivided into topics. You can select lessons in any order and you should follow the sequence in your training schedule so that both your on-the-job training and your Learndash theory training are in sync. You will be asked to complete knowledge check activities and quizzes as you proceed. You can stop at any time, as your progress will be saved.
This training course will cover all the theory that is necessary to support the training for your processing role and enable you to competently and confidently perform the many important tasks you have.
Some of the lessons will require you to read one of our Standard Operating Procedures (SOPs). Our SOPs have been created and refined over time to reflect best practice and many years of learning from our errors and improving our processes. Following our SOPs rigidly is vital to ensure the safety of the team and our wonderful donors and ensure the quality and safety of the blood products we manufacture and supply to be transfused into critically ill patients.
When you start the course you will be presented with the Course Content, which will show the lessons you will need to work through. Select the lesson you wish to take.
Your progress will be indicated by the progress bar at the top of the page.

You can navigate through the lessons and topics using the forward and backward buttons at the bottom of the page as shown or return to the course content.
Where it is present, you will need to click the Mark Complete button in some lessons to mark your progress.
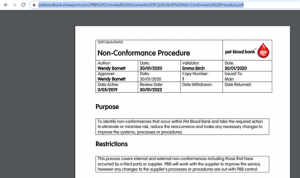
In order to read one of our controlled documents, when you click on the link you will be redirected to our secure document server ‘Sharepoint’. Use the log in details provided in your training schedule to access SharePoint.
To return to the lesson after reading the SOP just click on the back arrow (top left) as shown below.
Some of our interactive tasks may need you to decrease or increase your screen size so you can view everything.
At the end of the course there is a competency quiz with a pass mark of 100%. You have 2 attempts to pass the quiz. Please speak to the Training Department if you require further attempts.
Please don’t worry, this is an “open book” quiz so you can refer back to any of the documents from the course whilst you are answering the questions. Feel free to bookmark any of the SOPs as you go through the training, so that you can access them and their associated documents easily.
If you have any problems with your training please contact cpd@petbloodbankuk.org so we can help you.
Let’s get started!

Quality Department Update – February 2026
/in /by Alex Ashton
Welcome to this short update which covers recent changes to three SOPs related to canine blood donations.
If you have any queries about the training then please don’t hesitate to contact the Training Department at cpd@petbloodbankuk.org.
Donor Administration Department Update – February 2026
/in /by Alex Ashton
Welcome to this short update which covers recent changes to three SOPs related to canine blood donations.
If you have any queries about the training then please don’t hesitate to contact the Training Department at cpd@petbloodbankuk.org.
Permanent Collection Team Update – February 2026
/in /by Alex Ashton
Welcome to this short update which covers recent changes to three SOPs related to canine blood donations.
If you have any queries about the training then please don’t hesitate to contact the Training Department at cpd@petbloodbankuk.org.
Collection Team Update – February 2026
/in /by Alex Ashton
Welcome to this short update which covers recent changes to three SOPs related to canine blood donations.
If you have any queries about the training then please don’t hesitate to contact the Training Department at cpd@petbloodbankuk.org.
Donor Coordinator Course
/in /by Alex AshtonWelcome to your Donor Coordinator Training Course. Thank you for supporting us. This course has been put together to provide the theory training to augment your practical on-site training. It is vitally important that you work to our Standard Operating Procedures and to the principles of Good Manufacturing Practice (GMP) whilst working in your role.

If you are unsure of any of the training please do not worry and ask one of the team for help.
Work your way through the lessons as instructed by the Donor Admin Manager. To read a document click on the link which will take you to our secure document site.
Once you have read the document click on the back arrow in the top left-hand corner and this will bring you back to the Learndash lesson.

Once you have read the documents and completed any knowledge check exercises please move on to the next lesson. The training ends with a quiz which requires 100% pass mark. You have 2 attempts at the quiz but if you don’t pass please contact cpd@petbloodbankuk.org for assistance.

Out of Hours Induction Training (Retake)
/in /by Alex AshtonYou now have another 2 attempts to pass the quiz.
This isn’t a closed book test so feel free to revisit your original course and open the SOPs and INF documents so that you can refer to them during the quiz.
Good luck!
Human Resources Manager
/in /by Alex Ashton
Welcome to Pet Blood Bank, it is great you are joining us in our life-saving work.

As a Blood Bank we are governed by a branch of the government called the Veterinary Medicines Directorate or VMD. We have a long series of regulations we must adhere to in order to maintain our blood banking license and continue to save lives. Training is a large part of the VMD governance around which there are lots of rules.
This training course contains multiple lessons, some of which have been further subdivided into topics. You can select lessons in any order but you should begin with the Welcome and Induction lessons, then follow your training schedule so that both your on-the-job training and your Learndash theory training are in sync. You will be asked to complete knowledge check activities and quizzes as you proceed. You can stop at any time, as your progress will be saved.
This training course will cover all the theory that is necessary to support the training for your role and enable you to competently and confidently perform the many important tasks you have.
Some of the lessons will require you to read one of our Standard Operating Procedures (SOPs). Our SOPs have been created and refined over time to reflect best practice and many years of learning from our errors and improving our processes. Following our SOPs rigidly is vital to ensure the safety of the team and our wonderful donors and ensure the quality and safety of the blood products we manufacture and supply to be transfused into critically ill patients.
When you start the course you will be presented with the Course Content, which will show the lessons you will need to work through. Select the lesson you wish to take.

Your progress will be indicated by the progress bar at the top of the page.

You can navigate through the lessons and topics using the forward and backward buttons at the bottom of the page as shown or return to the course content.

Where it is present, you will need to click the Mark Complete button in some lessons to mark your progress.

In order to read one of our controlled documents such as an SOP, when you click on the link in the lesson you will be redirected to our secure document server ‘Sharepoint’. Use the log in details provided in your training schedule to access SharePoint.
To return to the lesson after reading the SOP just click on the back arrow (top left) as shown below.

Some of our interactive tasks may need you to decrease your screen size so you can view everything or increase it so you can view things clearly.
At the end of the course there will be a competency quiz that you have two total attempts to pass, the first attempt and then one retake. The pass mark is 100%. The quiz covers key areas of competency as identified by your Line Manager.
If you have any problems with your training please contact cpd@petbloodbankuk.org so we can help you.
Let’s get started!

Customer Service (Interim Manager John)
/in /by Tess CoakleyWelcome to your Course

This course provides the necessary training for the PBB processes that are part of your role.
Please move through the following lessons, reading any documents and completing any knowledge check questions or interactive exercises as you go.
When asked, please click on the document links in each lesson to be taken directly to the document. Once you have read the SOP click on the back arrow in the top left-hand corner to return to Learn Dash.
Please be aware that any recent changes to the SOPs will be highlighted in bold type on Scribe.
The course must be completed before you start you customer service role. The Course ends with a knowledge check quiz and a pass mark of 100% is required to complete the course. You will have a total of 2 attempts to pass the quiz and remember you can refer to the documents during the quiz, if you need to.
Any issues please email cpd@petbloodbankuk.org
Thank You
The Training Department
Out of Hours Dispatch Assistant ATR 2025 (Retake)
/in /by Alex Ashton
You now have a further 2 attempts to pass the quiz.
You still have access to your original course and can have all SOPs and INF documents open whilst completing your quiz.
Good Luck!
HR Onboarding Course
/in /by Tess CoakleyWelcome To Pet Blood Bank !
This your Human Resources onboarding induction course.
This course has been developed to guide you through essential HR topics and policies which help ensure a fair, safe, and supportive working environment.

If you have any queries regarding the training please contact the Training Department by emailing cpd@petbloodbankuk.org.
Work your way through the lessons. To read a document click on the link which will take you to our secure document site.
Once you have read the document click on the back arrow in the top left-hand corner and this will bring you back to the Learndash lesson.

Once you have read the documents and completed any knowledge check exercises move onto the next lesson. The training ends with a quiz which requires 100% pass mark. You have 2 attempts at the quiz but if you don’t pass please contact cpd@petbloodbankuk.org for assistance.
Out of Hours Dispatch Assistant Induction Training
/in /by Alex AshtonThank you for joining our team as an Out of Hours Dispatch Assistant. Your role is pivotal in ensuring that veterinary professionals can gain access to the life-saving blood products they need around the clock.
As a Blood Bank we are governed by a branch of the government called the Veterinary Medicines Directorate or VMD. We have a long series of regulations we must adhere to in order to maintain our blood banking license and continue to save lives. Training is a large part of the VMD governance around which there are lots of rules. One rule is the completion of the theory training and competency quiz prior to beginning the practical on-session’ training.
This training course contains multiple lessons, some of which have been further subdivided into topics. You can select lessons in any order but you should begin with the Welcome and Induction lessons, then follow your training schedule so that both your on-the-job training and your Learndash theory training are in sync. You will be asked to complete knowledge check activities and quizzes as you proceed. You can stop at any time, as your progress will be saved.
This training course will cover all the theory that is necessary to support the training for your role and enable you to competently and confidently perform the many important tasks you have.
Some of the lessons will require you to read one of our Standard Operating Procedures (SOPs). Our SOPs have been created and refined over time to reflect best practice and many years of learning from our errors and improving our processes. Following our SOPs rigidly is vital to ensure the safety of the team and our wonderful donors and ensure the quality and safety of the blood products we manufacture and supply to be transfused into critically ill patients.
There is no requirement to memorise the SOPs and you can have access to them during your quiz. An easy way to have them to hand is to open the SOPs via each LearnDash lesson. Once you have read the SOP then right click on the tab that the SOP appears in and select “Duplicate tab”. You can then leave that tab open and go back to the original tab and select the back arrow to return to LearnDash. This will leave all of the SOPs open in their individual web browser tabs so that you can easily access them as required. Alternatively you can create a “Favourite” for each SOP page and then they will stay in your Favourites menu so that you can click back on them as required.
When you start the course you will be presented with the Course Content, which will show the lessons you will need to work through. Select the lesson you wish to take.

Your progress will be indicated by the progress bar at the top of the page.

You can navigate through the lessons and topics using the forward and backward buttons at the bottom of the page as shown or return to the course content.

Where it is present, you will need to click the Mark Complete button in some lessons to mark your progress.

In order to read one of our controlled documents such as an SOP, when you click on the link in the lesson you will be redirected to our secure document server ‘Sharepoint’. Use the log in details provided in your training schedule to access SharePoint.
To return to the lesson after reading the SOP just click on the back arrow (top left) as shown below.

Some of our interactive tasks may need you to decrease your screen size so you can view everything.
If you have any problems with your training please contact cpd@petbloodbankuk.org so we can help you.
Let’s get started!

Out of Hours Dispatch Assistant Training for Existing Employees
/in /by Alex AshtonThank you for taking on the additional role of Out of Hours Dispatch Assistant.
As a Blood Bank we are governed by a branch of the government called the Veterinary Medicines Directorate or VMD. We have a long series of regulations we must adhere to in order to maintain our blood banking license and continue to save lives. Training is a large part of the VMD governance around which there are lots of rules. One rule is the completion of the theory training and competency quiz prior to beginning the practical on-session’ training.
This training course contains multiple lessons, some of which have been further subdivided into topics. You can select lessons in any order but you should begin with the Welcome and Induction lessons, then follow your training schedule so that both your on-the-job training and your Learndash theory training are in sync. You will be asked to complete knowledge check activities and quizzes as you proceed. You can stop at any time, as your progress will be saved.
This training course will cover all the theory that is necessary to support the training for your role and enable you to competently and confidently perform the many important tasks you have.
Some of the lessons will require you to read one of our Standard Operating Procedures (SOPs). Our SOPs have been created and refined over time to reflect best practice and many years of learning from our errors and improving our processes. Following our SOPs rigidly is vital to ensure the safety of the team and our wonderful donors and ensure the quality and safety of the blood products we manufacture and supply to be transfused into critically ill patients.
There is no requirement to memorise the SOPs and you can have access to them during your quiz. An easy way to have them to hand is to open the SOPs via each LearnDash lesson. Once you have read the SOP then right click on the tab that the SOP appears in and select “Duplicate tab”. You can then leave that tab open and go back to the original tab and select the back arrow to return to LearnDash. This will leave all of the SOPs open in their individual web browser tabs so that you can easily access them as required. Alternatively you can create a “Favourite” for each SOP page and then they will stay in your Favourites menu so that you can click back on them as required.
When you start the course you will be presented with the Course Content, which will show the lessons you will need to work through. Select the lesson you wish to take.

Your progress will be indicated by the progress bar at the top of the page.

You can navigate through the lessons and topics using the forward and backward buttons at the bottom of the page as shown or return to the course content.

Where it is present, you will need to click the Mark Complete button in some lessons to mark your progress.

In order to read one of our controlled documents such as an SOP, when you click on the link in the lesson you will be redirected to our secure document server ‘Sharepoint’. Use the log in details provided in your training schedule to access SharePoint.
To return to the lesson after reading the SOP just click on the back arrow (top left) as shown below.

Some of our interactive tasks may need you to decrease your screen size so you can view everything.
If you have any problems with your training please contact cpd@petbloodbankuk.org so we can help you.
Let’s get started!

Pet Blood Bank UK
Loughborough Technology Centre
Epinal Way
Loughborough
LE11 3GE
Call: 01509 232 222


